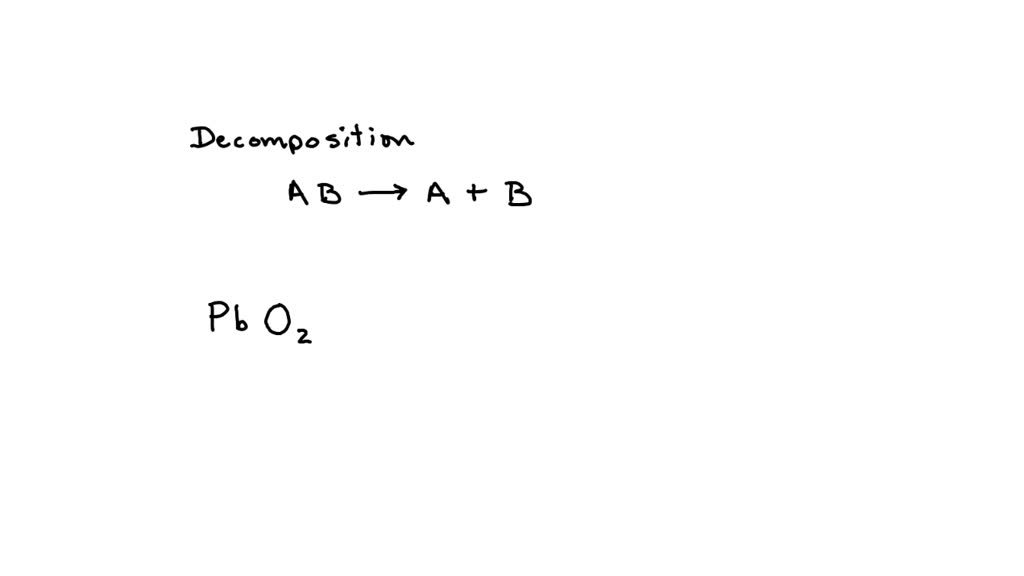Lead + Oxygen Equation . The one most commonly formed when it reacts with oxygen is lead (ii) oxide, also known. the following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide: 1 pbo = 1 pb + 1 o 2 for. lead + oxygen → lead oxide. let's balance this equation using the inspection method. when metals react with oxygen, metal oxides are formed. write a balanced chemical equation for the reactions given below: lead oxides are a group of inorganic compounds with formulas including lead (pb) and oxygen (o). The speed of the reaction depends on the reactivity of the metal. When hydrogen gas reacts is combined with oxygen gas and. First, we set all coefficients to 1: The lead is protected from further corrosion by the layer of lead oxide on the surface. lead can form many different oxides.
from www.numerade.com
1 pbo = 1 pb + 1 o 2 for. lead can form many different oxides. lead + oxygen → lead oxide. The lead is protected from further corrosion by the layer of lead oxide on the surface. when metals react with oxygen, metal oxides are formed. The speed of the reaction depends on the reactivity of the metal. the following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide: lead oxides are a group of inorganic compounds with formulas including lead (pb) and oxygen (o). First, we set all coefficients to 1: When hydrogen gas reacts is combined with oxygen gas and.
write a balanced equation to represent the of lead(IV
Lead + Oxygen Equation lead + oxygen → lead oxide. 1 pbo = 1 pb + 1 o 2 for. The speed of the reaction depends on the reactivity of the metal. The one most commonly formed when it reacts with oxygen is lead (ii) oxide, also known. when metals react with oxygen, metal oxides are formed. lead oxides are a group of inorganic compounds with formulas including lead (pb) and oxygen (o). the following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide: let's balance this equation using the inspection method. The lead is protected from further corrosion by the layer of lead oxide on the surface. lead can form many different oxides. write a balanced chemical equation for the reactions given below: When hydrogen gas reacts is combined with oxygen gas and. First, we set all coefficients to 1: lead + oxygen → lead oxide.
From www.numerade.com
Lead(II) oxide is obtained by roasting galena, lead(II) sulfide, in air Lead + Oxygen Equation When hydrogen gas reacts is combined with oxygen gas and. The speed of the reaction depends on the reactivity of the metal. lead + oxygen → lead oxide. First, we set all coefficients to 1: The lead is protected from further corrosion by the layer of lead oxide on the surface. The one most commonly formed when it reacts. Lead + Oxygen Equation.
From www.numerade.com
write a balanced equation to represent the of lead(IV Lead + Oxygen Equation let's balance this equation using the inspection method. when metals react with oxygen, metal oxides are formed. lead + oxygen → lead oxide. The one most commonly formed when it reacts with oxygen is lead (ii) oxide, also known. the following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide: The lead is. Lead + Oxygen Equation.
From brainly.in
Winkler method formula for dissolved oxygen analysis Brainly.in Lead + Oxygen Equation the following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide: First, we set all coefficients to 1: when metals react with oxygen, metal oxides are formed. The one most commonly formed when it reacts with oxygen is lead (ii) oxide, also known. lead can form many different oxides. lead + oxygen →. Lead + Oxygen Equation.
From www.nagwa.com
Question Video Describing How Oxidation Changes a Chemical Species in Lead + Oxygen Equation First, we set all coefficients to 1: 1 pbo = 1 pb + 1 o 2 for. write a balanced chemical equation for the reactions given below: lead can form many different oxides. When hydrogen gas reacts is combined with oxygen gas and. lead oxides are a group of inorganic compounds with formulas including lead (pb) and. Lead + Oxygen Equation.
From anaestheasier.co.uk
Alveolar Gas Equation Anaestheasier Lead + Oxygen Equation let's balance this equation using the inspection method. The speed of the reaction depends on the reactivity of the metal. First, we set all coefficients to 1: When hydrogen gas reacts is combined with oxygen gas and. 1 pbo = 1 pb + 1 o 2 for. when metals react with oxygen, metal oxides are formed. The one. Lead + Oxygen Equation.
From www.youtube.com
How to write Molecular formula of lead oxide Chemical formula of Lead Lead + Oxygen Equation lead oxides are a group of inorganic compounds with formulas including lead (pb) and oxygen (o). The lead is protected from further corrosion by the layer of lead oxide on the surface. lead + oxygen → lead oxide. lead can form many different oxides. write a balanced chemical equation for the reactions given below: The speed. Lead + Oxygen Equation.
From www.youtube.com
How to Write the Formula for Lead (IV) oxide YouTube Lead + Oxygen Equation When hydrogen gas reacts is combined with oxygen gas and. let's balance this equation using the inspection method. lead oxides are a group of inorganic compounds with formulas including lead (pb) and oxygen (o). lead can form many different oxides. lead + oxygen → lead oxide. write a balanced chemical equation for the reactions given. Lead + Oxygen Equation.
From www.chemistrylearner.com
Redox (OxidationReduction) Reaction Definition & Examples Lead + Oxygen Equation when metals react with oxygen, metal oxides are formed. lead can form many different oxides. the following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide: When hydrogen gas reacts is combined with oxygen gas and. write a balanced chemical equation for the reactions given below: 1 pbo = 1 pb + 1. Lead + Oxygen Equation.
From criticalcarenow.com
The Vitals Oxygen Delivery Equation CriticalCareNow Lead + Oxygen Equation write a balanced chemical equation for the reactions given below: the following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide: The one most commonly formed when it reacts with oxygen is lead (ii) oxide, also known. The lead is protected from further corrosion by the layer of lead oxide on the surface. when. Lead + Oxygen Equation.
From brainly.in
c. Lead dioxide [lead (IV) oxide]= Lead monoxide + Oxygen balance Lead + Oxygen Equation lead can form many different oxides. The one most commonly formed when it reacts with oxygen is lead (ii) oxide, also known. the following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide: The speed of the reaction depends on the reactivity of the metal. when metals react with oxygen, metal oxides are formed.. Lead + Oxygen Equation.
From www.researchgate.net
Simplified scheme for the oxygen evolution mechanism involving the Lead + Oxygen Equation lead + oxygen → lead oxide. let's balance this equation using the inspection method. the following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide: lead oxides are a group of inorganic compounds with formulas including lead (pb) and oxygen (o). lead can form many different oxides. The lead is protected from. Lead + Oxygen Equation.
From www.numerade.com
SOLVEDWrite the balanced chemical equation for each reaction. a. Solid Lead + Oxygen Equation The lead is protected from further corrosion by the layer of lead oxide on the surface. 1 pbo = 1 pb + 1 o 2 for. let's balance this equation using the inspection method. lead oxides are a group of inorganic compounds with formulas including lead (pb) and oxygen (o). When hydrogen gas reacts is combined with oxygen. Lead + Oxygen Equation.
From www.slideserve.com
PPT Reacting metals with oxygen PowerPoint Presentation, free Lead + Oxygen Equation write a balanced chemical equation for the reactions given below: When hydrogen gas reacts is combined with oxygen gas and. The lead is protected from further corrosion by the layer of lead oxide on the surface. First, we set all coefficients to 1: 1 pbo = 1 pb + 1 o 2 for. when metals react with oxygen,. Lead + Oxygen Equation.
From stock.adobe.com
Lead Sulfate PbSO4 molecule. Simple molecular formula consisting of Lead + Oxygen Equation The one most commonly formed when it reacts with oxygen is lead (ii) oxide, also known. First, we set all coefficients to 1: The speed of the reaction depends on the reactivity of the metal. lead oxides are a group of inorganic compounds with formulas including lead (pb) and oxygen (o). lead + oxygen → lead oxide. The. Lead + Oxygen Equation.
From pressbooks.lib.vt.edu
The Alveolar Gas Equation and AlveolarArterial PO2 Difference Lead + Oxygen Equation The lead is protected from further corrosion by the layer of lead oxide on the surface. 1 pbo = 1 pb + 1 o 2 for. when metals react with oxygen, metal oxides are formed. lead can form many different oxides. lead + oxygen → lead oxide. let's balance this equation using the inspection method. . Lead + Oxygen Equation.
From rk.md
Alveolar Gas Equation RK.MD Lead + Oxygen Equation lead oxides are a group of inorganic compounds with formulas including lead (pb) and oxygen (o). 1 pbo = 1 pb + 1 o 2 for. The lead is protected from further corrosion by the layer of lead oxide on the surface. write a balanced chemical equation for the reactions given below: lead can form many different. Lead + Oxygen Equation.
From www.toppr.com
In the of lead (ll) nitrate to give lead (ll) oxide Lead + Oxygen Equation The one most commonly formed when it reacts with oxygen is lead (ii) oxide, also known. the following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide: lead oxides are a group of inorganic compounds with formulas including lead (pb) and oxygen (o). lead + oxygen → lead oxide. write a balanced chemical. Lead + Oxygen Equation.
From brainly.in
write currently a balanced equation for the following word equation red Lead + Oxygen Equation the following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide: First, we set all coefficients to 1: lead + oxygen → lead oxide. let's balance this equation using the inspection method. write a balanced chemical equation for the reactions given below: when metals react with oxygen, metal oxides are formed. . Lead + Oxygen Equation.